Abstract

Dexamethasone is associated with a higher risk of infectious and non-infectious morbidity and mortality than prednisolone in induction therapy of children and young adults with Acute Lymphoblastic Leukaemia (ALL). We tested whether a 2 week induction dexamethasone schedule is less toxic than the standard 4 week schedule. Patients aged 1 -24 years with a new diagnosis of ALL or lymphoblastic lymphoma (excluding mature B lymphoma and ALL) in the UK and Ireland were randomly allocated to receive dexamethasone 5 mg/m2twice daily for 14 days (continuous in age <10 years and discontinuous week on/week off in age > 10 years) (Short) or 3 mg/m2 twice daily for 29 days followed by a 7 day wean (Standard). The primary end-point was a reduction in steroid related toxicity defined as any induction treatment related death, all serious adverse events (SAEs) within 8 weeks of the start of induction, and adverse events (AEs) which were classified as steroid related or contributory.
At diagnosis, patients were stratified by NCI (Standard Risk = age <10 and white cell count <50 x 109/l, High Risk = age > 10 years or white cell count >50x 109/l) and cytogenetic risk to receive one of 3 escalating intensity treatment regimens (A, B, C). NCI standard risk (SR) patients received a 3 drug induction (Regimen A) whilst high risk (HR) patients received 4 drugs including daunorubicin (Regimen B). Patients with poor risk cytogenetics were transferred to Regimen C. Minimal Residual Disease (MRD) was measured at the end of induction (day 29) and end of consolidation (week 14) using a Real Time Quantitative PCR technique with a sensitivity of 0.01%. Patients were classified as MRD low risk (day 29 <0.005%), intermediate risk (day 29 > 0.005% - 5%, week 14 <0.5%) or high risk (day 29 > 5% or week 14 >0.5%).
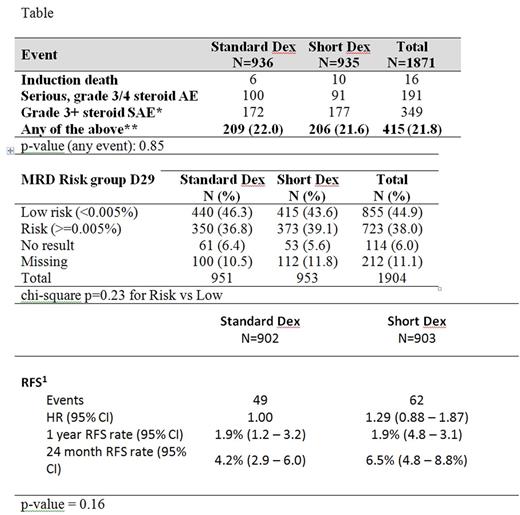
Between April 2012 and April 2017, 1904 eligible patients were randomly allocated to short (951) or standard dexamethasone (953) arms which were balanced for NCI risk, sex, immunophenotype and disease type (ALL or LBL). Analysis was intention to treat. Recruitment was stopped early on the recommendation of the data monitoring committee (IDMC) due to concerns about a non-significant excess of induction treatment related deaths in NCI standard risk patients with the short schedule (Short =9/503 (1.8%), Standard =4/506 (0.8%) (p =16) which led to a futility analysis demonstrating that there was <10% power of showing a significant improvement with short dexamethasone. At a median follow-up of 22.8 months, there is no statistically significant difference in steroid related toxicity, MRD response or relapse free survival between arms (Table).
A shorter induction schedule of dexamethasone 10 mg/m2/day x 2 weeks is no less toxic than 6 mg/m2/day x 4 weeks in children and young adults with ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal